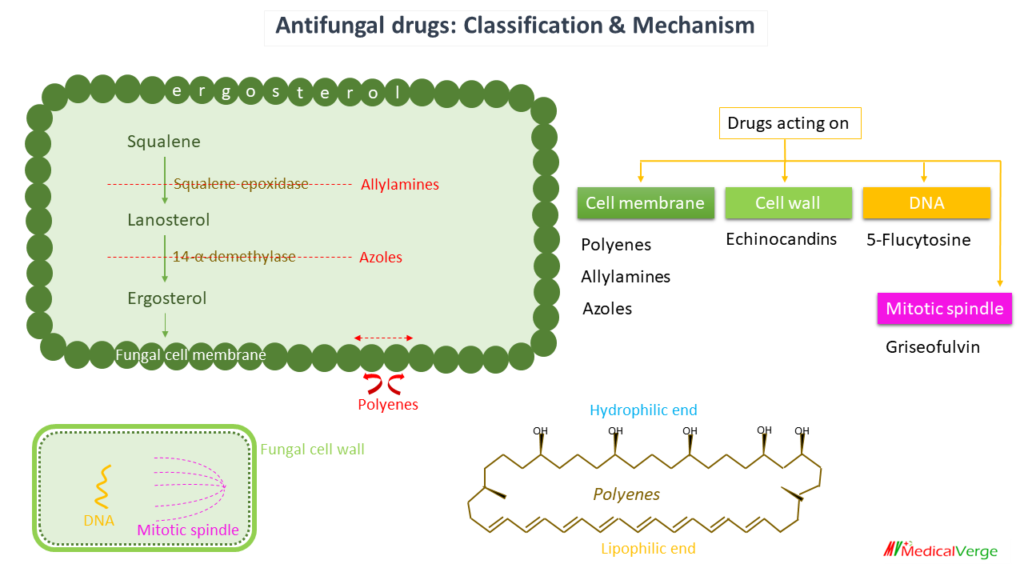
Antifungal drugs are used for superficial and deep/systemic fungal infections. Fungal infections are usually associated with the use of broad-spectrum antimicrobials, corticosteroids, anticancer or immunosuppressant drugs, indwelling catheters and implants, and emergence of AIDS. Saprophytic fungi can easily invade live tissue as a result of the aforesaid disruption of the host defence mechanisms. There are mainly 6 classes of antifungal drugs based on mechanism of action to use systemically as well as topically.
Polyenes
These are macrocyclic rings with one side that is highly lipophilic and has many conjugated double bonds and another side that is hydrophilic and has numerous OH groups. The polyenes have a strong affinity for the ergosterol found in the membrane of fungal cells. Polyenes combine with it and repel adjacent ergosterol molecules, result in formation of micropore to leak out intracellular material; fungicidal action.
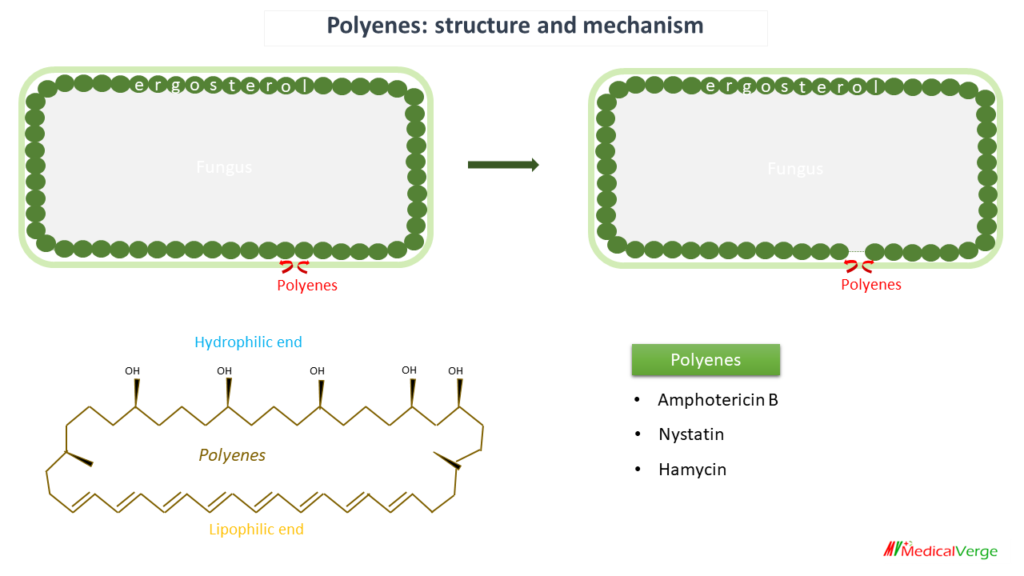
Amphotericin B is the drug of choice for all serious fungal infections, given by IV route. Side effects include infusion related reaction, nephrotoxicity and bone marrow suppression.
Cholesterol, present in host cell membranes, ergosterol closely resembles cholesterol, present in host cell membrane; the polyenes bind to it as well, though with lesser affinity. As a result, several side effects occur. AMB is one of the most toxic systemically used antibiotics. Polyenes have no antibacterial effect since they lack sterols.
- Amphotericin B
- Nystatin
- Hamycin
* Nystatin and hamycin are very toxic, hence used locally only.
* Liposomal preparation of amphotericin B has decreased risk of nephrotoxicity.
Allylamines
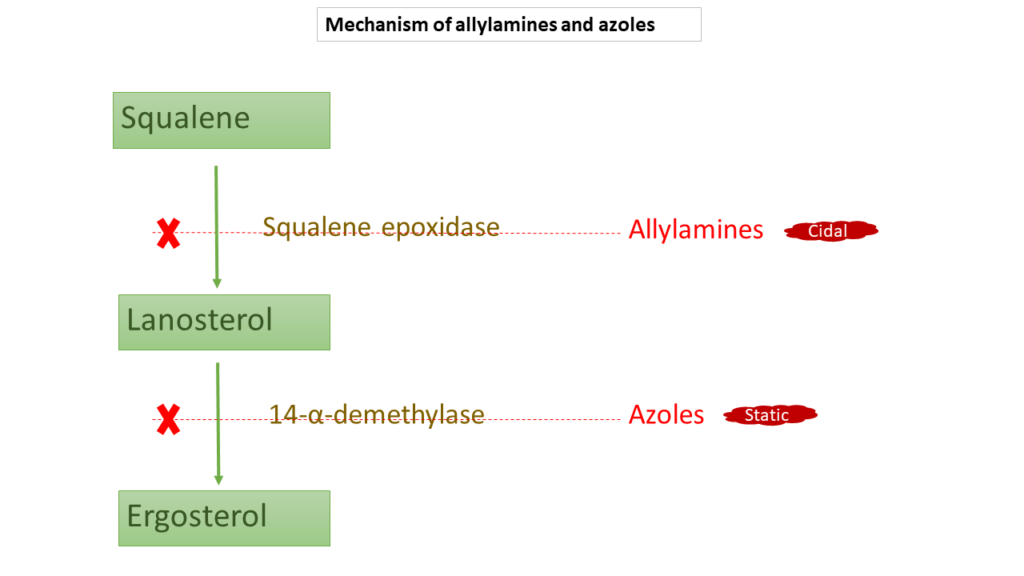
Allylamines act as a non-competitive inhibitor of the enzyme “squalene epoxidase,” which is involved in the initial step of fungal ergosterol synthesis. Accumulation of squalene which is toxic to fungal cells, responsible for the fungicidal action. Allylamines can be given orally but has very high affinity for keratin. After absorption, its reaches to only places where keratin is present (e.g., skin, hairs, nails). These are the places where drug can be applied directly, so used topically. Allylamines are drug of choice for dermatophytosis like tinea capitis, tinea pedis.
- Terbinafine
- Butenafine
- Naftifine
Azoles
These antifungal agents are currently the most widely used ones. They are broad spectrum, used as both topically and orally. Azoles inhibit 14-α-demethylase enzyme involved in ergosterol synthesis pathway. Unlike allylamines, lanosterol accumulate inside fungal cell which is not toxic to cell; hence fungistatic action.
- Ketoconazole
- Fluconazole
- Itraconazole
- Voriconazole
- Posaconazole
* Ketoconazole is no longer chosen for systemic use because of serious side effects like adrenal suppression, hepatotoxicity, gynecomastia and its strong tendency to inhibit microsomal enzymes.
* Fluconazole has highest bioavailability and max CNS penetration among azoles. It is DOC for mild to moderate candida and cryptococcal infection.
* Itraconazole given orally and DOC for histoplasmosis, blastomycosis and sporotrichosis (sporothrix).
* Voriconazole is DOC for aspergillosis.
* Posaconazole is the only effective azole for mucormycosis.
Echinocandins
These are a new class of potent semisynthetic antifungal antibiotics with a complex cyclic lipopeptide structure. it inhibits the synthesis of β-1, 3-glucan, which is an integral component of the fungal cell wall. As a result of the cell wall weakening, fungus cells become more vulnerable to osmotic pressure and eventually die. They are active mainly against Candida and Aspergillus. Strains of candida that have become resistant to azoles are susceptible to echinocandins.
- Caspofungin
- Micafungin
- Anidulafungin
Griseofulvin
Griseofulvin is chemically heterocyclic benzofuran. It was one of the early antibiotics extracted from Penicillium griseofulvum. clinical utility in dermatophytosis was demonstrated around 1960. It inhibits mitosis by interfering with mitotic spindle function.
Griseofulvin has high affinity for keratin like azoles. It is fungistatic for most dermatophytes but not against Candida and other fungi causing deep mycosis. Being deposited in the skin through circulation, it prevents fungal invasion of keratin. Because it is fungistatic rather than cidal, fungus persists in already infected keratin, till it is shed off. The newly formed keratin is not invaded by the fungus. Thus, the duration of treatment is dependent upon the site of infection, thickness of infected keratin and its turnover rate.
Griseofulvin is used orally. Because griseofulvin has a very low water solubility, its absorption from the GIT is a little erratic. Absorption is improved by taking it with fatty meal and by microfine preparation. It can cause disulfiram like reaction. It is not preferred now for dermatophytosis because of high chances of relapse.
5-Flucytosine
5-flucytosine (5-FC) is a narrow spectrum fungistatic agent. It is a prodrug, taken up by susceptible fungal cells and converted into active compound 5-fluorouracil (5-FU). Its chemical structure is similar to pyrimidine; competitively inhibit DNA polymerase. It is usually combined with amphotericin B for cryptococcal meningitis. Its synergistic action with AMB is utilized to reduce the total dose of the more toxic latter drug.