Therapeutic Drug Monitoring (TDM) is the measurement of drug and active metabolite concentrations in the plasma or serum to maximize therapeutic effect and minimize side effects. Although oral fluid (Saliva) drug concentration measurement is becoming popular in recent years and has been suggested as a non-invasive option, but it has a number of limitations.
Individualizing doses by keeping plasma drug concentrations within a target range (therapeutic range/therapeutic window) is attributed to as TDM. The therapeutic window is the range of plasma drug concentrations in which concentrations below the lower limit have no therapeutic effect and above the upper limit cause toxicity.
TDM is a multidisciplinary function. Accurate and clinically meaningful result can be obtained by collaboration among scientist, clinician, nurses and pharmacologist as the TDM team.
Criteria for therapeutic drug monitoring (TDM)
Characteristics of drugs suitable for TDM
- Narrow therapeutic index
- Marked inter-individual pharmacokinetic variability
- The desired therapeutic effect is difficult to monitor
- Direct correlation between plasma concentration of drug/metabolite and pharmacological effect
- Availability of appropriate analytical test/technique to determine drug or its metabolite level
Characteristics of drugs not suitable for TDM
- Wide therapeutic index, (e.g. beta-blockers, CCB)
- Pharmacological effect is clinically quantifiable (e.g. Antihypertensives by taking BP)
- Unestablished concentration-effect correlation
- Drugs for which, clinical outcome is unrelated to plasma concentration (e.g. hit & run drugs like PPI)
- There is no availability of appropriate technique to determine plasma drug level.
Common Drugs fulfilling TDM criteria
| S.N. | Class of drugs | Drugs |
| 1. | Antibacterials | Aminoglycosides – Amikacin, Gentamycin Glycopeptide – Vancomycin |
| 2. | Antiepileptics | Carbamazapine, Phenobarbitone, Phenytoin, Valproic acid |
| 3. | Antiarrhythmics | Digoxin, Digitoxin, Amiodarone, Lignocaine, Procainamide, Quinidine |
| 4. | Antidepressants | Tricyclic antidepressants |
| 5. | Bronchodilator | Theophylline |
| 6. | Cytotoxic drug | Methotrexate |
| 7. | Mood stabilizer | Lithium |
| 8. | Immunosuppressive | Cyclosporine |
Indications for TDM
1. Assessing compliance
To distinguish between inadequate drug treatment and non-compliance of the patient. TDM is done for determining the cause of therapeutic failure, if there is no apparent clinical response, despite being on appropriate dosage.
2. Diagnosing the condition of under-treatment
It is particularly important for prophylactic medications, such as antiepileptics, which are used to maintain the absence of a condition.
3. Toxicity
To prevent serious toxicity (e.g. when using aminoglycosides, cyclosporine) and to diagnose toxicity, when the clinical syndrome is undifferentiated (e.g. unexplained nausea in a patient taking digoxin)
4. Designing dosage regimen
When drug individualization or dose modification is needed, such as in the case of co-medication or co-morbidities (e.g., renal failure, advanced hepatic failure), or when a clinical endpoint is poorly defined.
5. Evaluating therapy
To evaluate treatment after a change in dosage regimen and a change in the patient’s clinical condition.
Therapeutic drug monitoring (TDM) process
TDM request
TDM would only be requested, when there is an appropriate indication. The following details must be communicated to the members of TDM team, with a drug assay request:
- Indication for monitoring
- Dosage regimen (dose, duration, dosage form)
- Pharmacokinetics and therapeutic range of the drug
- Co-medications and concomitant disease
- Patients’ demographic profile (age, sex, ethnicity)
- Timing of blood samples taken (Most Important)
Sample collection
Type of sample
1. Serum or Plasma
2. Oral fluid (Saliva) sampling for therapeutic drug monitoring is becoming more common, because it is a non-invasive alternative to serum. However, there are numerous limitations, and it can only be used for a limited number of drugs. The principle behind using saliva for TDM is that certain drugs diffuse passively and some drugs are actively secreted into the saliva. Drugs such as for phenobarbitone, phenytoin, carbamazapine and clobazam, salivary sampling may be used. The amount of a drug in saliva is proportional to the concentration of the free (unbound) drug, rather than the sum of bound and unbound drug measured in a plasma sample, which is a point of contention with salivary TDM. Intuitively, this should be preferred, since the free (unbound) fraction of the drug should reflect the amount of drug that is active in the body.
Timing of sampling
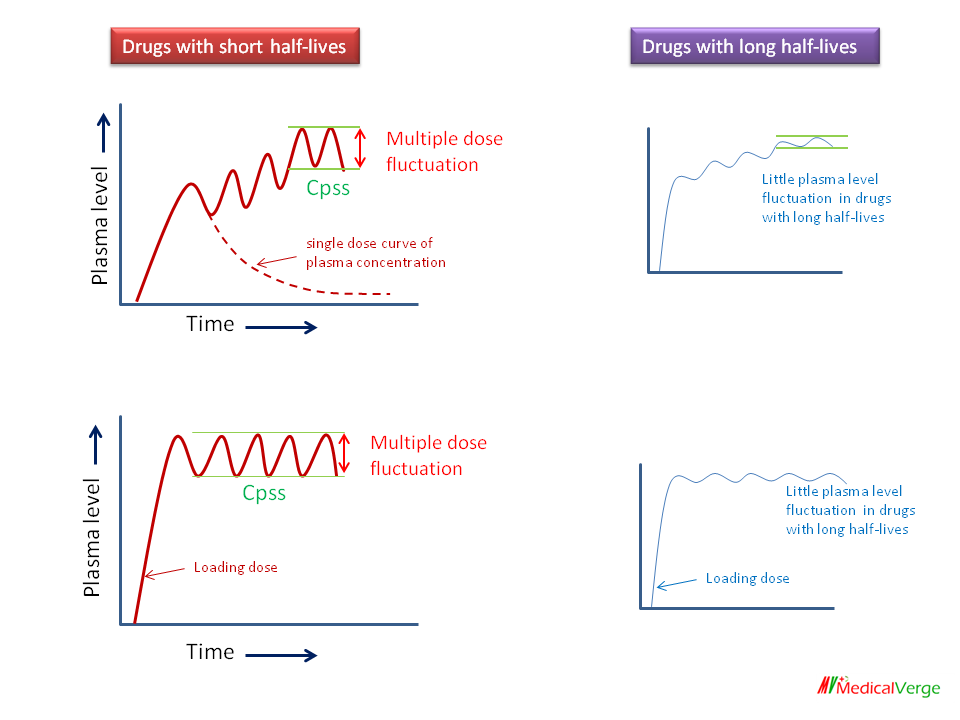
TDM sampling should be performed at steady state (Cpss) of plasma drug concentration, which occurs 4 to 5 half-lives after treatment begins. If a loading dose has been given, steady state can be reached earlier.
Since there is a small difference between peak and trough (lowest) plasma concentrations for drugs with long half-lives (t1/2), sampling can be performed at any time after 5 half-lives (e.g. Phenytoin).
Since there is a substantial difference between peak and trough plasma concentrations of drugs with short half-lives (e.g. Aminoglycosides), both are usually measured, pre-dose (just before the next dose) and post-dose. Gentamicin, for example, sample taken just before the administration and 0.5 hour after I.V. or 1 hour after I.M. administration.
Laboratory process
Analytical laboratories should ensure that, each analytical run has performed with the appropriate number of calibration standards and quality control. Assay performance is evaluated using an external quality assurance program wherever possible. Precision, sensitivity, and specificity of each assay should be documented and evaluated on a regular basis. An ideal analytical method would be capable of distinguishing between molecules with similar structures, detect small amounts, be unaffected by other drugs administered simultaneously, and be easy enough to use as a routine assay.
The following are some of the most widely used analytical techniques:
- ELISA (Enzyme-linked immunosorbent assay)
- FPIA (Fluorescence polarization immunoassay)
- RIA (Radioimmunoassay)
- TLC (Thin layer chromatography)
- HPLC & GLC (High-performance liquid chromatography & Gas liquid chromatography)
- Spectrometry and Fluorometry
Clinical application

Merits of therapeutic drug monitoring (TDM)
- Individualization of a patient’s medication dose regimen based on their health status or need.
- Optimizing the therapeutic benefit of a drug with a narrow therapeutic index while minimizing the adverse effects.
- In special circumstances, such as renal failure and advanced liver failure, it provide dosing assistance.
- Help in determining safe dosage long-term therapy and selecting an adequate initial dose to prevent therapy failure.
- It plays an important role in research and education, in understanding the concepts of rationale drug concentration and clinical issues highlighted by plasma drug concentration monitoring.
- TDM may improve the cost-benefit ratio by reducing hospital stays and drug therapy costs.
Demerits of TDM
- It is a resource consuming process that necessitates technological expertise and costly equipment.
- There may be laboratory variability in reporting.
- Limited accessibility or availability of TDM services
- TDM is only applicable to a small number of medications (those with a narrow therapeutic index).
- The population data for referring normal value is not available always